Vacc-C5 – a key component in a functional HIV cure
Introduction to the 5th constant (C5) domain of gp120
In the absence of antiretroviral therapy (ART), human immunodeficiency virus type 1 (HIV-1) infection will progress to AIDS in the majority of infected people, with the exception of rare long-term non-progressors (LTNP) or elite controllers (EC). LTNP and EC have low-level immune activation, as do patients on effective ART who are not or are very slowly progressing to AIDS. Immune activation arising from damage to the gut lumen leading to leakage of bacteria into the bloodstream is a prevailing concept. However, the gut is part of the lymph system and may, therefore, be influenced by antigens exposed by HIV – such as the C5 domain.
LTNP have high titer antibodies to the 5th constant (C5) domain on the HIV surface glycoprotein gp120, whereas rapid progressors have high titers to the V3 loop. Furthermore, loss of high titer antibodies to C5 immediately preceded progression to AIDS. The inability of anti-C5 antibodies to neutralize HIV infection in standard biological neutralizing assays may explain why the C5 region has not been considered for vaccine development.
We now know that conserved peptide domains such as those for Vacc-4x have to be conserved in order to stabilize the capsid (ref. Ref. Dahirel V et al. 2011 PNAS 08:11530-11535) in the same way there must a human entry / biological and / or viral fitness reason for the existence of the conserved C5 domain. The same applies to the “Kennedy epitope” (KE) else these domains would NOT be conserved.
Vacc-C5 description and mode of action
Previous studies that correlated the presence of antibodies to C5 with slow disease progression used a linear peptide antigen corresponding to the highly conserved carboxyterminal 15 amino acids from the C5 region for antibody detection. In virions, however, the C5 region interacts with gp41 to anchor gp120 to the membrane.
The Vacc-C5 construct consists therefore of 2 peptide domains; a.) the gp120-C5 domain and b.) the gp41-KE. The KE region is encompassing a stretch of highly conserved negatively charged residues that on the virion may form an electrostatic binding to the conserved positively charged residues on C5. This complex peptide, defined as Vacc-C5, induced antibodies in rabbits that cross-competed with HIV positive human sera from LTNP/natural viral suppressors (NVS). The antibody profiles derived from immunization of sheep and rabbits with this Vacc-C5 peptide are shown in Fig.1.a. and 1.b. below and show that 3 different epitopes can be developed leading to three possible modes of action scenarios alone or in combination.
- Antibodies binding to the C5 domain alone will cover up and block off the C5 sequence from exposure to the human cellular receptor(s) and thereby reduce immune activation. Reduced immune activation will lead to a lower production of virus and viral load. Furthermore binding to C5 will also block off interaction with gp41-KE and could destabilize the gp120 trimer sitting on top of its gp41-trimer and thereby lead to loss of the gp120 envelope resulting in a reduce infectivity and viral load.
- Antibodies binding to the gp41-KE will block off the interaction between gp41 and gp120 and could destabilize the gp120 trimer sitting on top of its gp41-trimer and thereby lead to loss of the gp120 envelope and a reduce infectivity and viral load.
- Antibodies binding to the complex between gp120-C5 and gp41-KE could prevent necessary structural changes needed for infectivity i.e. docking on the CD4 receptor and/or binding to the CCR5 ligand.The consequence of such prevention of structural changes could lead to neutralizing properties. Naturally – HIV in humans is less likely to form such antibodies because these 2 peptide domains are only bound together by electrostatic forces while in Vacc-C5 they are covalently coupled together. Consequently naturally developed antibodies binding only to the C5 domain will be the dominating mechanisms of actions in humans. This could be the explanation why no neutralizing properties have been associated with C5 antibodies.
- Antibodies binding to HIV during the budding process can also be part of the mode of action by Antibody-Dependent Cellular Cytotoxicity (ADCC).
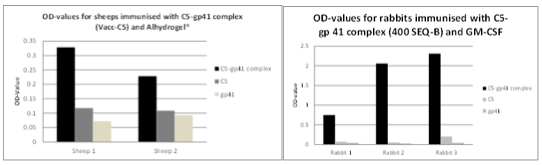
The figure above shows the 3 different antibody response profiles linked to the three mechanisms of action described.
Furthermore, to determine the clinical benefit of the mechanism of action anti-Vacc-C5 antibody levels were investigated in relation to disease progression using a cohort of archival sera from subjects at the Norwegian Institute of Public Health, from the time period 1989-1995 (prior to the advent of combination ART). Slow progressors (n=16) that lived for more than 6 years without ART had significantly higher levels of IgG (μg/ml) to the Vacc-C5 peptide than fast progressors, where the majority died within two years. The level of anti-Vacc-C5 antibodies was found to be stable over time for each individual, however, the time period for the progressors was shorter (2 years compared to 4-5 years). The 16 patients in the fast progressor group had a median anti-Vacc-C5 IgG of 0.39µg/ml, whereas the 16 slow progressors had a median of 6.70µg/ml. A cut-off of 2µg/ml was found to differentiate these two groups. Of the 16 fast progressors, only 2 out of 16 mounted an anti-Vacc-C5 IgG response above 2µg/ml plasma in contrast to the slow progressors, of which 14 of 16 patients had an anti-Vacc-C5 IgG level above 2µg/ml plasma. Viral load levels were re-tested after anti-Vacc-C5 status had been determined and remained relatively constant between the first and last time point tested. Ref. Cohort studies below.
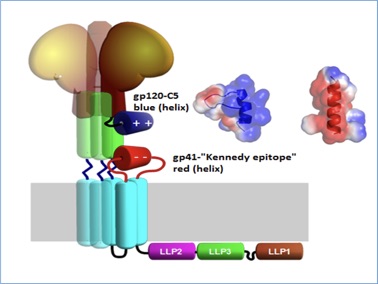
Figure 2 Illustration of gp120 (brown) and its C-terminal C5 region (blue) and its potential interaction with the gp41 C-helix(green) and the extracellular loop of gp41 (red). The trimeric nature of the complex is illustrated by shaded copies of the central parts. Inset shows electrostatic surface representation of the NMR structure of the C5-region (blue helix) and modeled helical structure of gp41 fragment 728 – 741 (red helix)
Overview of completed preclinical studies with Vacc-C5
R-HIV-2013-01 Vacc-HIV sheep study protocol
Vacc-4x, Vacc-C5 and Vacc-HIV vaccine all induce a peptide-specific serum IgG response directed against the components of the vaccine in sheep.
BPP03_001 Immunogenicity study of a vaccine against Human Immunodeficiency Virus (HIV)-1, Vacc-HIV, in rats.
Vacc-4x, Vacc-C5, and Vacc-HIV all induce both cellular and humoral immune responses in rats as measured by serum IgG, DTH and splenocyte IFN-γ production.
BPP03_004 Vacc-4x, Vacc-C5, Vacc-HIV (Vacc-4x + Vacc-C5 combined): 18 weeks intradermal administration immunogenicity study in the rabbit.
Vacc-C5 and Vacc-4x together with the local adjuvant GM-CSF can induce an antibody response in rabbit, alone or combined together in Vacc-HIV.
BPP03_005 Immunogenicity study of a vaccine against Human Immunodeficiency Virus (HIV)-1, Vacc-HIV in rat . Combined vs separate injections.
Vacc-4x and Vacc-C5 delivered separately or together as Vacc-HIV all induce both cellular and humoral immune responses in rats as measured by serum IgG and DTH.
Overview of completed Bionor-sponsored clinical trials with Vacc-C5
CTN-BI-Vacc-C5-2011/1
First in man; 36 patients, safety endpoint, Norway. Vacc-C5 with adjuvants GM-CSF or Alhydrogel safe and well tolerated.
https://clinicaltrials.gov/ct2/show/NCT01627678?cond=Vacc-C5+HIV&rank=1
Overview of completed Bionor-sponsored cohort studies with Vacc-C5
R-C5-2012-01 Analysis of anti-C5 antibody levels in an archival cohort of plasma samples from HIV-1 individuals with discrete clinical outcomes from the Norwegian Institute of Public Health.
Associated with slow disease progression in a treatment-naive historical longitudinal cohort from Norway.
R-C5-2012-02 Analysis of anti-C5 antibody levels in a cohort of plasma samples from HIV-1 individuals from the Institute of Human Virology’s clinics in Maryland, USA.
Correlated with moderate viral load (50 – 10,000 copies/ml) in a cohort including natural viral suppressors (NVS) in the United States.
R-C5-2012-03 Analysis of anti-C5 antibody levels in an archival cohort of plasma samples from HIV-1 individuals from Estonia.
Antibodies mapping to the C5 501–506 subdomain correlate with CD4 counts in treatment naïve HIV positive in an Estonian cohort.
Correlation of Antibody Responses to a Peptide Antigen gp120-C5501-512/gp41732-744 with HIV Disease Progression.
AIDS Res Hum Retroviruses. 2017 Jun;33(6):558-566. doi: 10.1089/AID.2016.0184. Epub 2017 Jan 31.
