A core component in a possible functional HIV cure – Vacc-4x
Vacc-4x is T-cell vaccine developed to guide the immune system to seek out and kill HIV-infected cells. The treatment aims to induce sustained immune responses to conserved /constant domains (regions of the virus common to all strains of HIV), which do not change despite mutations in other parts of the virus.

Vacc-4x is designed to induce CD4+ and CD8+ T cell responses that will remove HIV-infected cells, which should contribute to a reduction of the latent reservoir following re-activation by latency-reversing agents, such as romidepsin.
The following findings from Phase I and Phase II clinical trials of Vacc-4x provide the rationale for the company’s development program (ref. to clinical studies):
- It has been shown to reduce viral load statistic significantly (level of virus in the blood) and viral load set point by 0,64% equal to 0,44 log and that Vacc-4x can induce an immune response to HIV (CT BI-Vacc-4x 2007/1 clinical trial). (A subgroup of patients with naturally occurring C5 responses achieved greater VL reduction (-0.94 log / 88% )).
- Vacc-4x has been shown to induce long-term Vacc-4x specific T immune-cell memory indicating that Vacc-4x can induce durable immune responses (CTN BI-Vacc-4x/2009/1 and CT-BI Vacc-4x 2012/1 clinical trials).
- Immune responses to Vacc-4x have been shown to be safely re-stimulated indicating that periodic booster immunizations can be done to maintain immune effect as for vaccines in general (CTN BI-Vacc-4x/2009/1 and CT-BI Vacc-4x 2012/1 clinical trials).
- It has been shown to reduce viral pro-viral DNA, a measure of reservoir size, in cART treated patients, indicating that HIV infected latent reservoir cells are killed, also in the presence of cART (CT-BI Vacc-4x 2012/1 clinical trials).
- Vacc-4x has demonstrated reduction of plasma HIV-RNA after reactivation of latent reservoir, indicating immune-based killing of HIV containing CD4+ T cells (BPC01-001 clinical trial (REDUC)).
- To date, Vacc-4x has been shown to be safe and well tolerated, also in combination with other treatments (cART, romidepsin, lenalidomide) (BPC01-001 clinical trial (REDUC)). Bionor completed the REDUC clinical trial in December 2015 evaluating whether priming the immune system with Vacc-4x to attack HIV infected CD4+ T cells upon activation of the latent reservoir can improve viral control after administration of the latency reversing agent, romidepsin. REDUC with Vacc-4x and romidepsin successfully met its primary endpoint by significantly reducing latent HIV reservoir and demonstrated control of viral load. The headline results in REDUC were:
- The latent HIV reservoir was significantly reduced by 40% (Total HIV DNA and qVOA).
- Viral load remained below the level of detection in 11 out of 17 patients on combination antiretroviral therapy (cART) despite reservoir reactivation. Four patients had measurable but low viral load and only at one of the three romidepsin infusions.
- The pharmacodynamic effect of romidepsin, i.e., reactivation of the latent HIV reservoir, was confirmed by increases in histone acetylation levels and viral expression.
- The combination of Vacc-4x and romidepsin was safe and well tolerated
Vacc-4x description and mechanism of action
Vacc-4x consists of 4 peptides corresponding to the conserved regions on the p24 protein, ref. Fig1.a. These regions must be highly conserved in order to maintain viral fitness.
These Vacc-4x peptides are from the common hexamer interface and for the HIV to maintain replication fitness functional hexamers (buildings blocks) are a prerequisite for the HIV capsid.
Mutations in or immunological pressure from a vaccine like Vacc-4x on these interfaces will lead to nonfunctional hexamers with a defect capsid and an HIV that is less or unable to replicate, ref. Fig1.b.
In general, immune responses to p24 and to Gag proteins (structural proteins that include p24) are associated with slow disease progression and improved viral control. Conserved regions represent functionally critical regions on the virus where mutations are likely to affect virus replication competence and viability
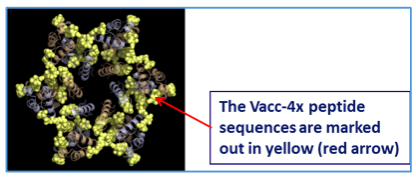
Fig1.a: The location of the Vacc4x peptides in the p24hexamer. Note how the yellow-labeled peptide sequences are overlapping the RED/GREEN areas on the hexamer and the GREEN interface sequences in Fig.1.b. below. The immune system of HIV controllers target these areas.
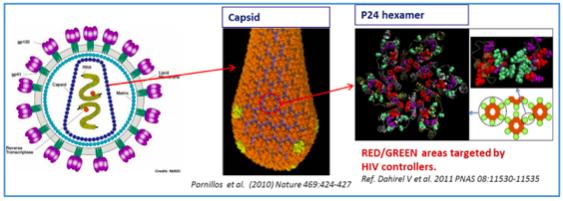
Fig.1.b: The figures show the link between; HIV-Capsid-p24Hexamer and the p24Hexamer interfaces
By using 4 peptides Vacc-4x it will increase the potential for immune recognition of infected cells in diverse human populations. Furthermore, the 4 peptides in Vacc-4x are modified using proprietary technologies such as amino acid substitution, addition and deletion to facilitate and enhance uptake by dendritic cells.
The Vacc-4x vaccine is intended to induce, sustain or recover CD4+ and CD8+ T cell responses to p24, so that the immune cells recognize and remove infected and HIV producing cells presenting the native versions of such p24 peptides.
Overview of completed Bionor-sponsored clinical trials with Vacc-4x
| CTN B-HIV-1/99 | First in man; 11 patients, 0.4 mg, safety endpoint, Norway. |
| CTN B-HIV-2/2001 | Dose finding, 40 patients, 0.4 mg and 1.2 mg, immunogenicity, Norway. |
| CT BI-Vacc-4x 2007/1 | Efficacy and safety, 135 patients, 1.2 mg, reduction in viral load setpoint after treatment interruption, first placebo controlled, randomised, double-blind study, U.S., UK, Germany, Spain, Italy. |
| CTN BI-Vacc-4x/2009/1 | Re-boost of 25 of the 38 patients from CTN B-HIV-2/2001, 1.2 mg, Norway. |
| CT-BI Vacc-4x/IMiD-2010/1 | Vacc-4x and lenalidomide, 36 patients, randomised, double blind, 1.2 mg Germany. |
| CTN-BI/Vacc-4x/L3-2011/1 | Intranasal randomized controlled immunogenicity study on 24 patients with modified Vacc-4x Gag peptides with Endocine as adjuvant. Results showed Vacc-4x proliferative T cell responses increased only among the vaccinated (p ≤ 0.031). |
| CT-BI Vacc-4x 2012/1 | Re-boost of 33 patients from CT BI-Vacc-4x 2007/1, 1.2 mg, U.S., UK, Germany, Spain, Italy. |
| BPC01-001 (REDUC) | Vacc-4x and romidepsin, 26 patients, open label, 1.2 mg, Denmark. |
